Abstract
Introduction: Donor lymphocyte infusion (DLI) has been used as a preemptive therapy for those with mixed donor chimerism (MDC) or in the presence of minimal residual disease or as salvage therapy for relapse of disease post allogeneic stem cell transplantation (allo SCT). The efficacy of DLI and the ease of therapy are balanced by the potential for significant toxicity with 100-day DLI-related mortality rate approaching 20%. A number of strategies have been employed in an effort to avoid the major complication of DLI, namely Graft-versus-host disease (GVHD). These strategies include the use of escalating doses of T cells (1 x 10 6 - 1 x 10 8/ kg) and manipulations of the T cells prior to infusion. We conducted a retrospective study of the prophylactic role of low dose DLI (< 5 x 10 6 /kg) in patients with mixed chimerism in the setting of in-vivo T cell depleted allo SCT.
Methods: We identified 33 consecutive patients who underwent allo SCT with T cell depletion conditioning regimen with a minimum of 100-day post-transplant follow up. Five patients qualified for our study. MDC was defined by CD3 or CD33 subsets <=95% of the donor and trending down in two or more consecutive samples measured at 100-day or beyond. Full donor chimerism (FDC) was > 95% of CD3 or CD33 subsets. We adopted two myelo-ablative and 1 reduced intensity conditioning with in-vivo T cell depletion in all regimens. Myelo-ablative regimens: Fludarabine 30 mg IV day -8 to day -4 and Busulfan 3.2 mg IV from day -5 to day -2. GVHD prophylaxis consisted of anti-thymocyte globulin for a total dose of 7.5 mg/kg IV from day -4 to day -2 and tacrolimus starting day -1 (FB-ATG regimen). Carmustine 300 mg/ m2 IV day -6, Cytarabine 400 mg/ m2 and etoposide 200mg/ m2 IV on day -5 to day -3, melphalan 140 mg/ m2 IV on day -2. GVHD prophyalxis consisted of alemtuzumab 20 mg IV on day -5 to day -2 and tacrolimus starting day -1 (BEAM-C regimen). Reduced intensity regimen consisted of Fludarabine 30mg/m2 on day -6 to day -2 and melphalan 140 mg/ m2 on day -2. GVHD prophylaxis consisted of alemtuzumab 20 mg IV daily from day -8 to day -5 and tacrolimus starting day -1 (FMC regimen). Demographic, disease and transplant variables were collected.
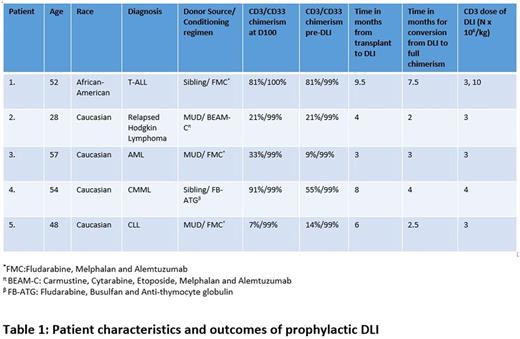
Results: The median age was 52 (range: 28-57) years. There was 1 patient each with diagnoses of T-cell Acute Lymphoblastic Leukemia, Acute Myelogenous Leukemia, Relapsed Hodgkin Lymphoma, Chronic Lymphocytic Leukemia, and Chronic Myelomonocytic Leukemia. Three patients had received FMC regimen and 1 each received FB-ATG and BEAM-C regimen. All five patients had MDC in CD3 subset and FDC in CD33 subset pre-DLI. The median CD3 chimerism pre-DLI was 21% (9%-81%). None of the patients were diagnosed with acute or chronic GVHD pre-DLI. The median time to DLI infusion was 6 (3-9.5) months. The median CD3 dose of the DLI was 3 (range: 3-10) x 106 /kg. The median time for conversion of MDC to FDC was 3 (2-7.5) months. All 5 patients converted to FDC in CD3 subset. One out of five patients needed a second DLI (CD3 cell dose: First DLI 3 x 106 /kg and second DLI 10 x 106 /kg) due to persistent MDC in CD3 subset (Table 1). One patient (20%) developed steroid responsive acute GVHD of the skin, GI and liver (grade III) following DLI (CD 3 dose 3 x 106 /kg) and this has evolved into extensive cGVHD which is under partial remission. At 1 year follow up: none relapsed and the OS was 100%.
Conclusion: Low dose DLI (CD 3 dose starting at 3-4 x 106 /kg) is highly effective (80%) as a prophylactic therapy for conversion of MDC to FDC with minimal risk of GVHD in the setting of in-vivo T cell depleted allo SCT.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal